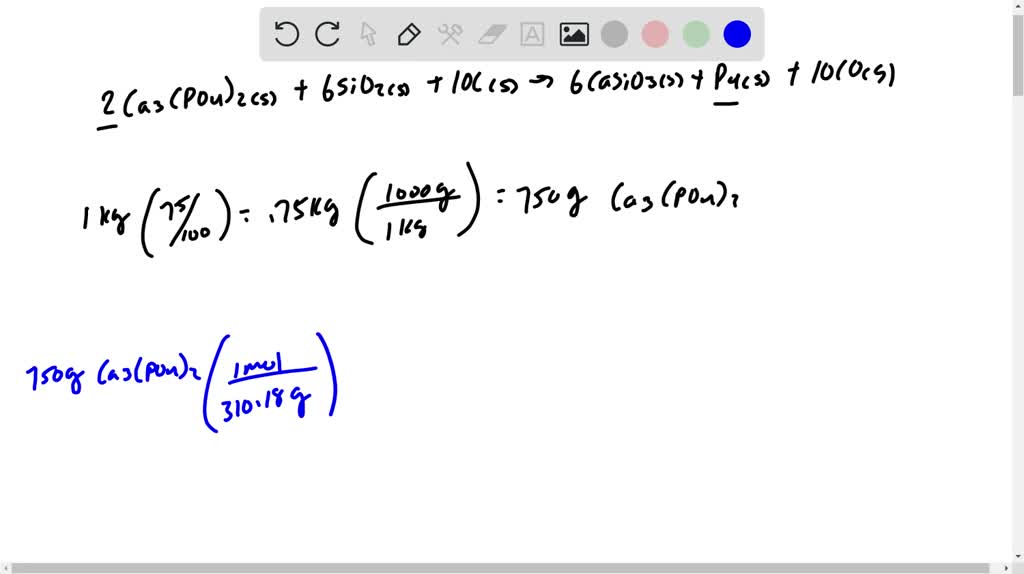
SOLVED:Phosphorus can be prepared from calcium phosphate by the following reaction: 2 Ca3(PO4)2(s)+6 SiO2(s)+ 10 C(s) ⟶ 6 CaSiO3(s)+P4(s)+10 CO(g) Phosphorite is a mineral that contains Ca3(PO4)2 plus other non-phosphorus-containing compounds. What

SOLVED: The desired product is calcium phosphate. How is the calcium phosphate collected after the reaction is completed? A. Filtration B. Evaporation C. Distillation D. Decantation A 1.520 g sample containing a












![PDF] Solubility of calcium phosphates. | Semantic Scholar PDF] Solubility of calcium phosphates. | Semantic Scholar](https://d3i71xaburhd42.cloudfront.net/526753a222f5c73710b1769a7ab93c423a92aa0f/5-Table1-1.png)